POPC:POPE:CHOL bilayer (Coarse-Grained).
Last executed: Jan 3rd, 2024 with MDAnalysis 2.7.0 and Python 3.11
Packages required: MDAnalysis, NumPy.
Packages for visualization: Matplotlib, SciPy.
In this tutorial, we are going to use MembraneCurvature to derive surfaces and calculate curvature of a lipid bilayer of lipid composition POPC:POPE:CHOL and a 5:4:1 ratio from a Molecular Dynamics (MD) simulation carried out using the Martini force field.
import MDAnalysis as mda
from membrane_curvature.base import MembraneCurvature
from membrane_curvature.tests.datafiles import MEMB_GRO, MEMB_XTC
import matplotlib.pyplot as plt
import numpy as np
%matplotlib inline
MDAnalysis : INFO MDAnalysis 2.7.0 STARTED logging to 'MDAnalysis.log'
MDAnalysis : INFO MDAnalysis 2.7.0 STARTED logging to 'MDAnalysis.log'
MDAnalysis : INFO MDAnalysis 2.7.0 STARTED logging to 'MDAnalysis.log'
Because the system included in this tutorial uses the Martini coarse grain representation, we prefer to suppress the warnings from MDAnalysis. Please note that this shouldn’t be the standard approach for your code as warnings may give you some heads-up about issues with your system.
import warnings
warnings.filterwarnings('ignore')
For a better understanding of how MembraneCurvature help us to calculate membrane curvature, let’s split the process into five main steps:
1. Load MDA Universe
We start by loading our molecular dynamics output files (coordinates and trajectory) into an MDAnalysis Universe:
universe = mda.Universe(MEMB_GRO, MEMB_XTC)
A quick check of our Universe gives us a better idea of the system used in this tutorial. To check the dimensions of the simulation box, number of lipid residues, total number of beads, as well as the number of frames in the trajectory contained in our Universe, we can run:
print("Universe info:")
print("\nBox dimensions[x,y,z]: {}." \
"".format(universe.dimensions[:3]))
print("\n{} lipid residues and {} beads." \
"".format(universe.residues.n_residues, universe.residues.n_atoms))
print("\nLipid types: ")
[print("-"+lipid) for lipid in set(universe.residues.resnames)]
print("\nThe trajectory includes {} frames."\
"".format(universe.trajectory.n_frames))
Universe info:
Box dimensions[x,y,z]: [241.12502 241.12502 233.69261].
2046 lipid residues and 23736 beads.
Lipid types:
-POPC
-CHOL
-POPE
The trajectory includes 11 frames.
It probably gets easier if we can have a quick visual inspection of the system. To visualize our system, we can make use of the NGL Viewer.
import nglview as nv
def color_by_resname(system, zoom, color_, box=False):
view = nv.show_mdanalysis(system)
view.add_representation('ball+stick', selection='not CHOL', radius=0.5, color="grey")
view.add_representation('ball+stick', selection='.PO4', radius=2.00, color=color_)
view.add_representation('ball+stick', selection='not POPC and not POPE', radius=0.5, color="yellow")
view.add_representation('ball+stick', selection='.ROH', radius=2.00, color="orange")
if box == True:
view.add_unitcell()
view.camera='orthographic'
view.control.zoom(zoom)
view.control.rotate(
mda.lib.transformations.quaternion_from_euler(
-np.pi/2, np.pi/3, np.pi/12, 'rzyz').tolist())
return view
color_by_resname(universe, 0.5, "brown")
The NGL Viewer enables us to identify two main types of lipids: phospholipids, POPC and POPE lipids, shown in grey colour; and sterols, CHOL lipids, shown in yellow. All phospholipid head groups are shown in red, and sterol head groups are shown in orange.
Since membraneCurvature uses the dimensions of the box to set the dimensions of the grid (See Algorithm), it is important to run a preliminary check to confirm that our system does not display significant box size fluctuations over time.
We can quickly check the fluctuations of our simulation box for the x and y dimensions with:
for ts in universe.trajectory:
print(ts.frame, ts.dimensions[:2])
0 [241.12502 241.12502]
1 [241.33353 241.33353]
2 [240.43678 240.43678]
3 [240.13823 240.13823]
4 [240.14366 240.14366]
5 [240.20067 240.20067]
6 [239.7728 239.7728]
7 [240.65321 240.65321]
8 [239.9748 239.9748]
9 [240.36978 240.36978]
10 [240.5304 240.5304]
With a fluctuation of $~1%$ in each dimension, we know that the grid set by MembraneCurvature using the u.dimensions will contain all the elements in our system.
Now that we have a better idea of what the system used in this tutorial contains and we have verified the fluctuations of the box are not significant, we can go to step 2 and select our atoms of reference.
2. Select Atoms of Reference
The MembraneCurvature algorithm uses an AtomGroup as a reference to derive surfaces. Therefore, it’s important to identify what constitutes a sensible selection for an AtomGroup of reference. Keep in mind that choosing atoms that display flip-flop during your simulation is not a good idea. The atoms in your AtomGroup of reference should remain in the same leaflet over the simulation time.
Typically in lipid bilayers, phospholipid headgroups are the most straightforward AtomGroup from which to derive surfaces. In this tutorial, we are going to choose phospholipid headgroups of phosphate beads. In the Martini representation, these beads are called PO4. Then, we can select the phospholipid head groups (PO4 beads) in our Universe with:
PO4_headgroups = universe.select_atoms('name PO4')
In contrast to sterols, phospholipids molecules have a very slow flip-flop rate. As a general approach, phospholipid head groups are a better choice than sterol headgroups. Hence, our `AtomGroup` does not include cholesterol headgroups (`ROH` beads) and uses `PO4` beads instead.
The atoms selected in PO4_headgroups include the whole membrane instead of the individual leaflets within which the PO4_headgroups reside. Since MembraneCurvature calculates curvature from a single surface, we should select an AtomGroup for each leaflet.
To select leaflets we have two options:
2.1 Direct parsing
If we know which atoms or beads belong to each leaflet, we can directly parse the selection. It is recommended to check that your selection is correct before running MembraneCurvature.
In this tutorial, we are working with a lipid bilayer that can be split into the upper and the lower leaflet based on their residue number and the name of the head group. We know that we have 1023 residues per leaflet, and since we are going to derive surfaces based on this selection, we also choose the lipid headgroups.
So we can select our leaflets with:
upper_leaflet = universe.select_atoms('resid 0-1023 and name PO4')
print('We have {} beads in the upper leaflet'.format(len(upper_leaflet)))
lower_leaflet = universe.select_atoms('resid 1024-2046 and name PO4')
print('and {} beads in the upper leaflet'.format(len(lower_leaflet)))
We have 921 beads in the upper leaflet
and 921 beads in the upper leaflet
To double-check that the variables lower_leaflet and upper leaflet contain atoms from different leaflets, we write a function using NGL Viewer to visualize the AtomGroup for each leaflet according to the selection above.
def color_by_leaflet(system, zoom, box=False):
view = nv.show_mdanalysis(system)
view.add_representation('ball+stick', selection='0-1023 and .PO4', radius=2.00, color="steelblue")
view.add_representation('ball+stick', selection='1024-2046 and .PO4', radius=2.00, color="darkcyan")
if box == True:
view.add_unitcell()
view.camera='orthographic'
view.control.zoom(zoom)
view.control.rotate(
mda.lib.transformations.quaternion_from_euler(
-np.pi/2, np.pi/3, np.pi/12, 'rzyz').tolist())
return view
color_by_leaflet(universe, 0.5)
From the NGL widget we can identify two leaflets. The upper leaflet, coloured in blue, with residues 1-1023; and the lower leaflet, coloured in cyan, with residues 1024-2046.
2.2 Leaflet identification
We can automatically identify lipids by leaflet using the leaflet MDAnalysis module. This option may be very helpful when we don’t know the details of our system and we need to identify the residues in each leaflet of the bilayer.
To identify leaflets using the MDAnlysis LeafletFinder analysis module we do:
from MDAnalysis.analysis.leaflet import LeafletFinder
L = LeafletFinder(universe, 'name PO4')
up_leaflet = L.groups(0) # upper leaflet
low_leaflet = L.groups(1) # lower leafet
Here, the variables up_leaflet and low_leaflet are AtomGroups.
These selections are equivalent to the previous ones stored in the variables upper_leaflet and lower_leaflet. We can run a quick check to verify the selections are the same with:
up_leaflet == upper_leaflet
True
low_leaflet == lower_leaflet
True
In this tutorial, we are going to use the selection obtained from LeafletFinder. We can print the range of residues for each leaflet and the total number of elements with:
leaflets = ["Lower", "Upper"]
list_leaflets = [up_leaflet.residues.resids,
low_leaflet.residues.resids]
for name, lf in zip(leaflets,list_leaflets):
print("{} leaflet includes resid {}-{} " \
"and a total of {} elements.".format(name,
lf[0],
lf[-1],
len(lf)))
Lower leaflet includes resid 103-1023 and a total of 921 elements.
Upper leaflet includes resid 1126-2046 and a total of 921 elements.
The selections obtained by LeafletFinder can be visualized with the color_ by_leaflet function as well:
color_by_leaflet(up_leaflet + low_leaflet, 0.5)
Now that we know how to select our AtomGroup of reference, we can run MembraneCurvature.
3. Run MembraneCurvature
MembraneCurvature is a Python class that performs multiframe analyses to derive surfaces from the AtomGroup of reference. From the derived surface, MembraneCurvature calculates mean and Gaussian curvature per frame, and their respective average over frames.
MembraneCurvature(universe, # universe
select='name PO4', # selection of reference
n_x_bins=12, # number of bins in the x dimension
n_y_bins=12, # number of bins in the y_dimension
wrap=True) # wrap coordinates to keep atoms in the main unit cell
To use MembraneCurvature, we have four main parameters to provide:
Universe: The Universe that contains our system of interest. In this tutorial, our Universe comprises a membrane of POPC POPE CHOL lipids, as described in Section 1.
Atom selection (
select): This is a key parameter to run MembraneCurvature. Based on this selection, surfaces will be derived for every frame in the trajectory. Simultaneously, curvature will be calculated from the derived surface. In this tutorial, we selected each one of the leaflets in our Universe as shown in 2. Select Atoms of Reference.
Number of bins (
n_x_bins,n_y_bins): This parameter will determine how many bins are assigned to the grid in each dimension. In Membrane Curvature, the dimensions of the grid are determined by the size of the simulation box contained in the Universe. The grid comprisesn_x_binsxn_y_binsnumber of bins. Choosing the number of bins is also important. Choosing too many bins may introduce undefined values in the grid built by the MembraneCurvature algorithm. On the other hand, a very low number of bins may result in significant loss of information. We recommend taking bins of size ~20 Å. In Section 1 we found that our Universe has dimensions ~240 x 240 Å. Therefore, in this tutorial we are going to usen_x_bins=n_y_bins=12.Coordinate wrapping (
wrap): Applying coordinate wrapping is useful when we have atoms in our simulation box falling outside the boundaries of the simulation box. Since this is a raw trajectory, and we want to have a high number of lipid headgroups to derive the surface, we usewrap=Trueto put all the atoms in the primary unit cell.
Now that we have have the AtomGroup associated to each leaflet, and we know what parameters are needed, we can run MembraneCurvature. To run MembraneCurvature on the upper leaflet we do:
curvature_upper_leaflet = MembraneCurvature(universe,
select="resid 103-1023 and name PO4",
n_x_bins=12,
n_y_bins=12,
wrap=True).run()
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting analysis loop over 11 trajectory frames
MDAnalysis.analysis.base: INFO Starting analysis loop over 11 trajectory frames
MDAnalysis.MDAKit.membrane_curvature: WARNING Atom coordinates exceed size of grid and element (12,5) can't be assigned. Maximum (x,y) coordinates must be < (241.12501525878906, 241.12501525878906). Skipping atom.
MDAnalysis.MDAKit.membrane_curvature: WARNING Atom coordinates exceed size of grid and element (12,5) can't be assigned. Maximum (x,y) coordinates must be < (241.12501525878906, 241.12501525878906). Skipping atom.
MDAnalysis.analysis.base: INFO Finishing up
MDAnalysis.analysis.base: INFO Finishing up
Similarly, for the lower leaflet, we run MembraneCuvature with:
curvature_lower_leaflet = MembraneCurvature(universe,
select='resid 1024-2046 and name PO4',
n_x_bins=12,
n_y_bins=12,
wrap=True).run()
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting analysis loop over 11 trajectory frames
MDAnalysis.analysis.base: INFO Starting analysis loop over 11 trajectory frames
MDAnalysis.analysis.base: INFO Finishing up
MDAnalysis.analysis.base: INFO Finishing up
Note:
The default MDAnalysis .run() includes all the trajectory frames included in the Universe. To calculate membrane curvature in specific frames, you can pass specific frames or steps by calling run using the start, stop, step parameters.
For example, to calculate curvature in the upper leaflet for the 4th and 7th frame, we run:
curv_upper_leaflet_f_4_and_7 = MembraneCurvature(universe,
select='resid 1-1023 and name PO4',
n_x_bins=12,
n_y_bins=12,
wrap=True).run(start=4, stop=8, step=3)
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Choosing frames to analyze
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting preparation
MDAnalysis.analysis.base: INFO Starting analysis loop over 2 trajectory frames
MDAnalysis.analysis.base: INFO Starting analysis loop over 2 trajectory frames
MDAnalysis.analysis.base: INFO Finishing up
MDAnalysis.analysis.base: INFO Finishing up
4. Extract Results
MembraneCurvature stores the calculation of surface and curvature in the .results attribute. We can find two different types of results:
Multiframe
We can extract the data from MembraneCurvature for all the frames in the trajectory. These results are available for surface, mean and Gaussian curvature.
curvature_upper_leaflet.results.z_surface
curvature_upper_leaflet.results.mean_curvature
curvature_upper_leaflet.results.gaussian_curvature
Average over frames
We extract values of surface, mean and Gaussian curvature averaged over then_framesof the trajectory.
curvature_upper_leaflet.results.average_z_surface
curvature_upper_leaflet.results.average_mean
curvature_upper_leaflet.results.average_gaussian
In this section, we are going to extract the results for surface, mean and Gaussian curvature. However, to illustrate the two types of results you can obtaine with MembraneCurvature, the example of the derived surface will use the Multiframe result, and for curvature we will show results when it is averaged over frames.
4.1 Surface
As we mentioned before, the array containing the derived surface for each frame in the trajectory can be found in the results attribute.
We can check the surface derived from the lipid headgroups in the upper leaflet with:
surface_upper_leaflet = curvature_upper_leaflet.results.z_surface
It’s important to note that surface_upper_leaflet is an array of shape (n_frames, n_x_bins, n_y_bins). Since the results extracted here correspond to the multiframe analyses, the values in the grid contains the averaged Z coordinate value of the lipid headgroups for a given bin, and for every frame.
We can check the shape of the results for surface doing:
surface_upper_leaflet.shape
(11, 12, 12)
This shape indicates that we have 11 frames in our trajectory, and for each frame we have a surface derived from our AtomGroup. It also indicates that the dimensions of our simulation box was divided into 12 bins.
Similarly, for the lower leaflet we run:
surface_lower_leaflet = curvature_lower_leaflet.results.z_surface
Now that we know how to extract the calculated surface and curvature values, the final step is to visualize the results. To visualize multiframe results, go to visualizing multiframe results.
4.2 Curvature
The resulting curvature values are stored in the attributes .results.average_mean_curvature and
results.average_gaussian_curvature.
These arrays contain the computed values of mean ($H$) and Gaussian ($K$) curvature averaged over the n_frames of the trajectory. The units of mean curvature is 1/lenght (1/Å), and the units of Gaussian curvature is 1/[length^2] (1/Å$^{2}$).
Mean Curvature ($H$)
We can save the calculated values of membrane curvature for each leaflet in two new variables called mean_upper_leaflet and mean_lower_leaflet:
mean_upper_leaflet = curvature_upper_leaflet.results.average_mean
mean_lower_leaflet = curvature_lower_leaflet.results.average_mean
In this case, since we are extracting the results of averaged mean curvature, the shape of these arrays is simply (n_x_bins, n_y_bins):
mean_upper_leaflet.shape
(12, 12)
Gaussian Curvature ($K$)
Since the information of the averaged Gaussian curvature is stored in the results.average_gaussian attribute, we can store the values of $K$ in another two variables gaussian_upper_leaflet and gaussian_lower_leaflet:
gaussian_upper_leaflet = curvature_upper_leaflet.results.average_gaussian
gaussian_lower_leaflet = curvature_lower_leaflet.results.average_gaussian
Now that we have extracted the data containing values of surface, mean and Gaussian curvature, we can proceed to visualize the results.
Note:
Running MembraneCurvature analysis iterates over the trajectory provided in the Universe. In this particular example, our Universe has 11 frames. By running MembraneCurvature from a static file (i.e. coordinates file only with no trajectory), the data stored in the average over frames results (results.average_z_surface, results.average_mean, and results.average_gaussian) will be the same as the Multiframe results (results.z_surface, results.mean, results.gaussian).
5. Visualize Results
There are two main approaches to visualize results from MembraneCurvature.
Plotting MembraneCurvature results using contours or imshow requires setting the correct array orientation.
To plot results from the membrane curvature analysis, use the transposed array np.array([...]).T, and place the [0, 0] index of the array in the lower left corner of the axes by setting origin='lower'.
Please check the examples below for more details.
Contours
To visualize the results obtained from the MembraneCurvature we can use contourf from Matplotlib. By using contourf to plot MembraneCurvature results, we gather all the points of equal value and the region enclosed by that set of points is coloured according to a colormap of preference.
To illustrate the most basic plot we can generate using contours, we are going to plot the averaged mean curvature obtained for the upper leaflet. We do:
fig, ax = plt.subplots(1,1)
# plot contour with transposed arrays and set the origin to [0,0] using origin=lower
ax.contourf(mean_upper_leaflet.T, cmap='bwr', origin='lower', linewidth=0.1)
ax.set_aspect('equal')
ax.set_title('Basic plot: $H$ Upper Leaflet')
plt.show()
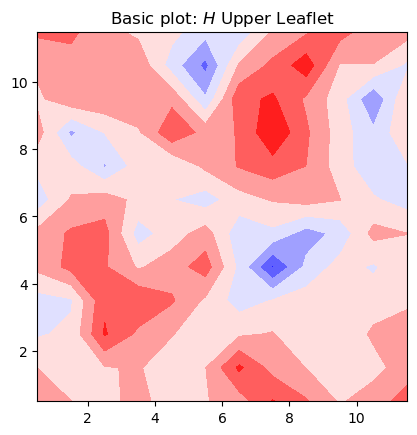
In this basic plot of mean curvature, there are several rough edges in the contours, and it lacks a colorbar for reference.
A more elaborate way to plot contours uses the zoom function from the scipy.ndimage package for multidimensional processing. With the zoom option, we smooth the contours.
Hence, a smooth contour plot of mean curvature with its respective color bar can be generated with the function plot_contours as follows:
from scipy import ndimage
def plot_contours(results, label, levels_, cm):
"""
Function used to plot contours of MembraneCurvature results.
User can determine number of contour lines / regions (levels),
label of the colorbar (label) and colormap (cmap).
Parameters
----------
results: list
List with results by leaflets as elements [lower_leaflet, upper_leaflet]
label: str
Label to add to colorbar.
levels: int
Determines number of contour lines.
cmap: str
Colormap to use in plot.
"""
fig, [ax1, ax2] = plt.subplots(ncols=2, figsize=(4,3.5), dpi=200)
max_ = np.max(abs(results[0]))
for ax, rs, lf in zip((ax1, ax2), results, leaflets):
rs = ndimage.zoom(rs, 3, mode='wrap', order=2)
if np.min(rs) < 0 < np.max(rs):
levs = np.linspace(-max_, max_, levels_)
im = ax.contourf(rs.T, cmap=cm, origin='lower', levels=levs, alpha=0.95, vmin=-max_, vmax=max_)
tcs = [-max_, 0, max_]
else:
levs = np.linspace(int(np.min(rs)), round(np.max(rs)), levels_)
im = ax.contourf(rs.T, cmap=cm, origin='lower', levels=levs, alpha=0.95, vmin=int(np.min(rs)), vmax=round(np.max(rs)))
tcs = [int(np.min(rs)), round(np.max(rs))]
ax.set_aspect('equal')
ax.set_title('{} Leaflet'.format(lf), fontsize=6)
ax.axis('off')
cbar = plt.colorbar(im, ticks=tcs, orientation='horizontal', ax=ax, shrink=0.7, aspect=10, pad=0.05)
cbar.ax.tick_params(labelsize=4, width=0.5)
cbar.set_label(label, fontsize=6, labelpad=2)
return
Note that there are three arguments in the plot_contours function. The first one is a list that contains the results of curvature by leaflet, the second one is the label to attach to the colorbar, the third one is the number of levels (see contourf documentation), and the fourth one is the colormap to use in the plot.
Then, to make use of plot_contours, we also need to define a list of the results by leaflet, as well as the name of each leaflet:
mean_curvs = [mean_lower_leaflet, mean_upper_leaflet]
We can generate a more elaborated contour plots for mean curvature with:
plot_contours(mean_curvs, "$H$ ($\AA^{-1}$)", 50, "bwr")
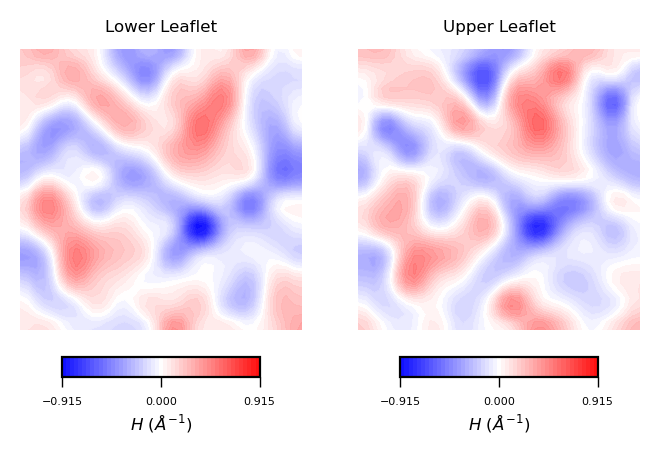
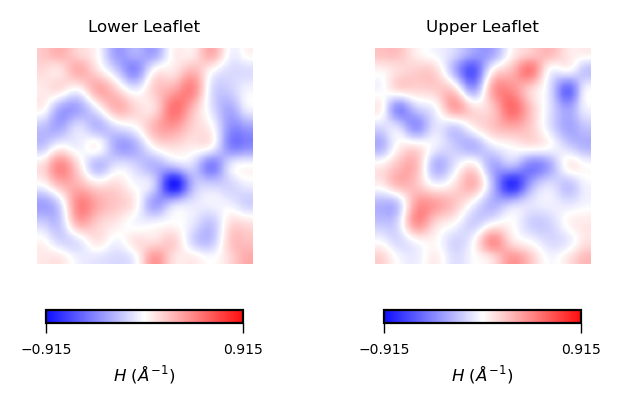
Interpreting mean curvature plots is straightforward. Mean curvature ($H$) gives information about the “inverted shape” of the surface. Positive mean curvature indicates valleys (red coloured), negative mean curvature indicates peaks (blue coloured).
Hence, from the obtained contour plot of mean curvature we can identify:
Coupling between leaflets. Regions of positive curvature (red coloured) in the lower leaflet match those in the upper leaflet. The same is observed for regions of negative mean curvature (blue coloured).
A central region of negative mean curvature. For both upper and lower leaflet, there is a central region of negative mean curvature (blue coloured) along the
yaxis, while regions of positive mean curvature (red coloured) are localized at the corners of the membrane, in particular bottom left and upper right corners.
Similar to the approach used for mean curvature, to plot the results of averaged Gaussian curvature we first create the list of curvatures:
gaussian_curvs = [gaussian_lower_leaflet, gaussian_upper_leaflet]
And then call the plot_contours function:
plot_contours(gaussian_curvs, "$K$ ($\AA^{-2}$)", 30, "PiYG")
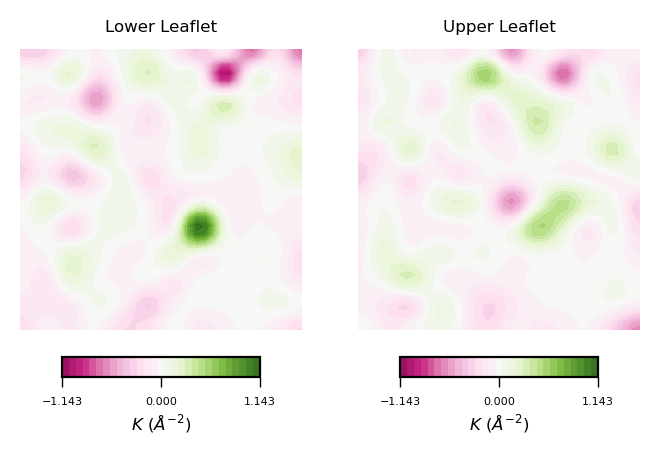
To read plots of Gaussian curvature a rule of thumb is: negative Gaussian curvature represents saddles, positive Gaussian curvature represents concave regions. Generally speaking, Gaussian curvature is associated to the elasticity of the membrane.
From the obtained contour plot of Gaussian curvature, we can identify:
Regions of positive Gaussian curvature (coloured in green) dominate in both leaflets.
Overall, the membrane display concave regions (green coloured regions). In particular in the lower leaflet, we can identify a spot with the highest values of $K$. This can be translated to high flexibility of the membrane during the simulation.
For a nice graphical review on curvature, including mean and Gaussian curvature, check this link.
Imshow
As an alternative, we can also plot results from MembraneCurvature via imshow. By using imshow, the visualization is generated by plotting each element of the array in a matrix of m x n elements and according to a colormap of reference. In the case of MembraneCurvature, the matrix has the same shape as the arrays stored in the .results attributes. So it will be a matrix of n_x_bins, n_y_bins. The color of each square is determined by the value of the corresponding array element and the color map used.
The most basic plot using imshow to plot the surface in the lower leaflet can be obtained by:
fig, ax = plt.subplots(1,1)
ax.imshow(surface_upper_leaflet[0].T, cmap='YlGnBu_r', origin='lower')
ax.set_aspect('equal')
ax.set_title('Basic plot: Surface Upper Leaflet')
plt.show()
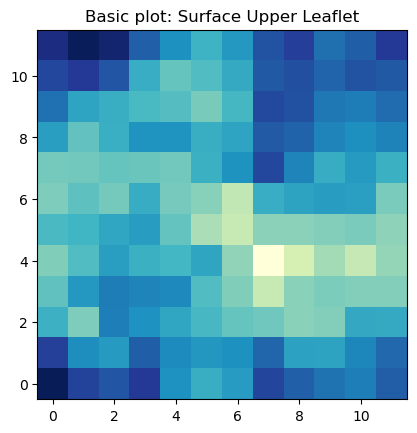
The basic imshow plot to visualize the surface in the upper leaflet show a different color for each bin in the array. From this plot is very easy to identify we have 12 bins in each dimension.
This plot, however, is not visually pleasing. We can improve an imshow plot by adding an interpolation method. For consistency with the contour plots, I am going to use the 'gaussian' inteprolation method. For more inteprolation methods you can read the imshow interpolation Matplotlib docs.
Hence, we can generate better imshow plots by doing something like:
surfaces = [surface_lower_leaflet[0], # surface in lower leaflet from frame=0
surface_upper_leaflet[0]] # surface fn upper leaflet from frame=0
fig, [ax1, ax2] = plt.subplots(ncols=2, figsize=(4,2), dpi=200)
for ax, surfs, lf in zip((ax1, ax2), surfaces, leaflets):
im = ax.imshow(surfs.T, interpolation='gaussian', cmap='YlGnBu_r', origin='lower')
ax.set_aspect('equal')
ax.set_title('{} Leaflet'.format(lf), fontsize=6)
ax.axis('off')
cbar = plt.colorbar(im, ticks=[surfs.min(), surfs.max()], orientation='horizontal', ax=ax, shrink=0.7)
cbar.set_ticklabels([int(surfs.min()), int(surfs.max())])
cbar.ax.tick_params(labelsize=5, width=0.5)
cbar.set_label("Height lipid headgroups (${\AA}$)", fontsize=6, labelpad=2)
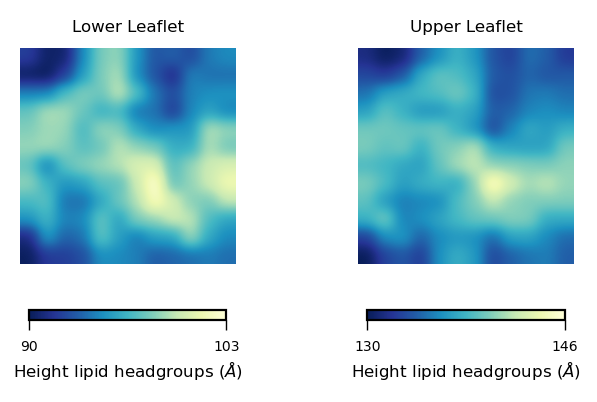
In this plot we can see the height of the lipid headgroups in the lower (left panel) and upper leaflet (right panel). The colormap allows us to identify the heighest values of the lipid headgroups, coloured in yellow. The lowest values are shown in blue.
From these plots, we can identify a “bump” in the middle region of the membrane (yellow coloured), which is reduced progressively towards the edges of the simulation box.
Visualizing multiframe results
We can take advantage of the the multiframe analysis extracted for the membrane surfaces performed in section 4.1 to visualize how the surface of our membrane changes over frames:
def plot_over_frames(surfaces, lf, label, cmap):
fig, axs = plt.subplots(ncols=10, figsize=(8,4), dpi=300)
for ax, surfs, frame in zip(axs, surfaces, range(universe.trajectory.n_frames)):
im = ax.imshow(surfaces[frame].T, interpolation='gaussian',
cmap=cmap, origin='lower',
vmin=surfaces.min(), vmax=surfaces.max())
ax.set_aspect('equal')
ax.set_title('frame={}'.format(frame), fontsize=6)
ax.axis('off')
cbar = plt.colorbar(im, ticks=[surfs.min(), surfs.max()],
orientation='vertical', ax=axs, shrink=0.2,
aspect=15, pad=0.01)
cbar.set_ticklabels([int(surfs.min()), int(surfs.max())])
cbar.ax.tick_params(labelsize=5, width=0.5)
cbar.set_label(label, fontsize=3, labelpad=2)
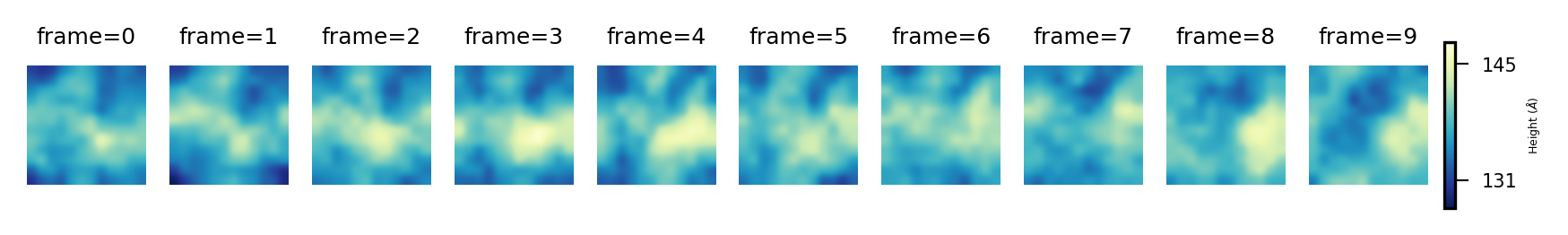
Then, we can get the plots of the derived surface in the upper leaflet, over the 11 frames of the trajectory by:
plot_over_frames(surface_upper_leaflet, # surfaces by frame
'Upper', # Leaflet name
"Height (${\AA}$)", # Colorbar label
'YlGnBu_r') # cmap
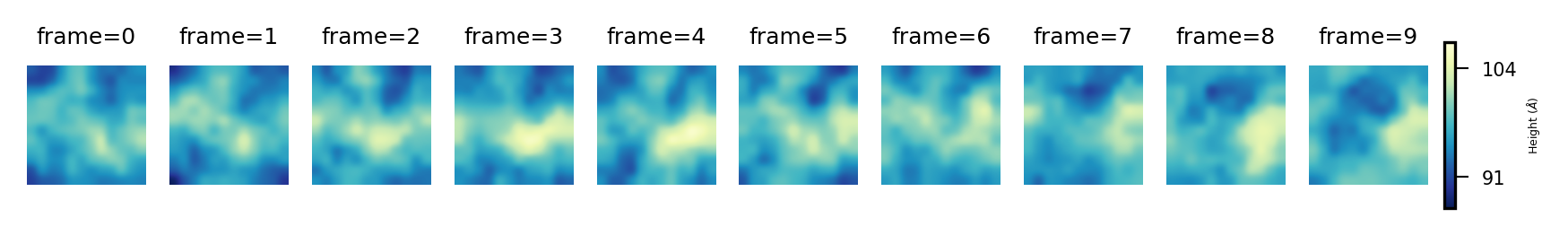
And for the lower leaflet:
plot_over_frames(surface_lower_leaflet ,
'Lower',
"Height (${\AA}$)",
'YlGnBu_r')
To plot curvature using imshow, we can define a function to plot each leaflet based on this plot:
def plot_by_leaflet(results, label, cmap):
fig, [ax1, ax2] = plt.subplots(ncols=2, figsize=(4,2), dpi=200)
max_ = np.max(abs(results[0]))
max_
for ax, rs, lf in zip((ax1, ax2), results, leaflets):
rs = ndimage.zoom(rs.T, 3, mode='wrap', order=2)
im = ax.imshow(rs, interpolation='gaussian', cmap=cmap, origin='lower', alpha=0.95, vmin=-max_, vmax=max_)
ax.set_title('{} Leaflet'.format(lf), fontsize=6)
ax.axis('off')
cbar = plt.colorbar(im, ticks=[-max_, max_],
orientation='horizontal', ax=ax, shrink=0.7,
aspect=15)
cbar.ax.tick_params(labelsize=5, width=0.5)
cbar.set_label(label, fontsize=6, labelpad=2)
return
Then, using a similar approach as for the results.z_surface, and using our handy function plot_by_leaflet, we can plot the mean curvature associated to each leaflet
mean_curvs = [mean_lower_leaflet, mean_upper_leaflet]
plot_by_leaflet(mean_curvs, "$H$ ($\AA^{-1}$)", "bwr")
With the stored variables gaussian_upper_leaflet and mean_upper_leaflet, we can plot the results for the Gaussian curvature ($K$) using the function plot_by_leaflet.
gaussian_curvs = [gaussian_lower_leaflet, gaussian_upper_leaflet]
plot_by_leaflet(gaussian_curvs, "$K$ ($\AA^{-2}$)", "PiYG")
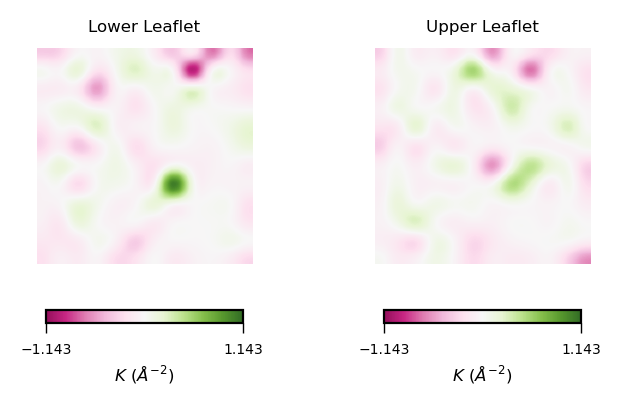
Et voilá :)